 by Véto-pharma
by Véto-pharma In 2014, Véto-pharma launched its experimental apiary, a cornerstone of its intensive research and development efforts. This apiary, together with an ad hoc R&D laboratory set up the following year, has significantly bolstered Véto-pharma’s capabilities in combating varroa mites.
By 2018, our innovation team of engineers, researchers, and beekeeping technicians had refined molecule screening methods, accelerating the journey from testing new active ingredients to the launch of breakthrough medications. Over a hundred active ingredients have been evaluated, aiming to develop safe and effective treatments for varroa mites that protect bee colonies, users, and consumers alike.
When will a new product be available on the market? This is by far the most common question received but the answer is complex as the path from discovery to market is intricate, laden with regulatory hurdles and complex safety requirements. While the urgency for new treatments is high, each step—from efficacy discovery to drug registration—demands rigorous testing, particularly to mitigate toxicity risks. Essential oils, despite their efficacy, often prove harmful to bees at optimal doses. Moreover, securing sufficient live varroa mites for testing remains a significant challenge, one that our experimental apiary addresses adeptly.

The screening project will continue until we fully validate one or more active ingredients for their effectiveness against varroa mites and their safety for bees. Currently, these validations are conducted mainly in vitro or in bee cages. Once an active ingredient passes the screening stage, it enters into a preclinical development to define its pharmacological, pharmacokinetic, and toxicological properties. Next, it moves to clinical development, where the final formulation is chosen and tested in clinical trials. The registration process begins after clinical validation and the preparation of a Marketing Authorisation (MA) dossier. On average, these stages take 5 to 10 years before the medicine is ready for beekeepers to use.
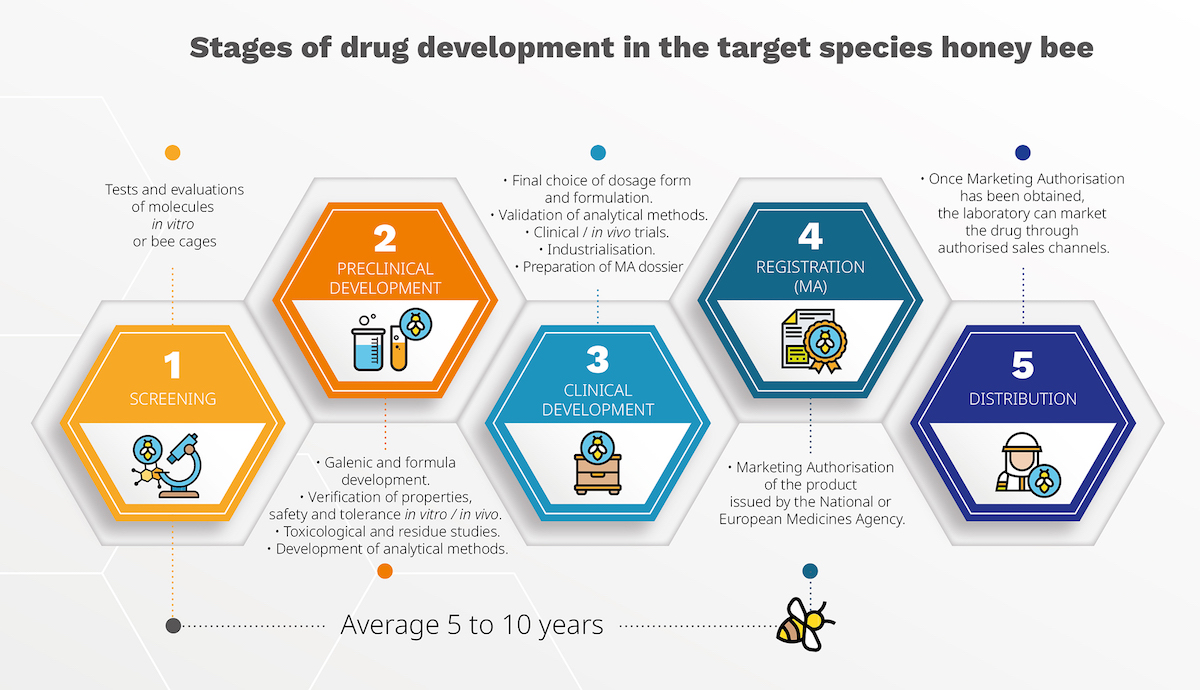
Molecules undergo extensive testing to evaluate their chemical and pharmacological properties, identifying those with therapeutic potential. Véto-pharma’s tailored methods ensure the selection of compounds that are both effective and minimally toxic to bees.

This stage involves creating the drug’s formulation, galenic and analytic development, and assessing its pharmacological properties, safety, and tolerance in bees. Comprehensive toxicology and residue studies are also conducted.

Upon finalizing the formulation, clinical trials are conducted to confirm the drug’s efficacy under real-world conditions (field studies). These trials are crucial for demonstrating therapeutic benefits and are compiled into a Marketing Authorisation Application (MAA).

Post-clinical validation, a Marketing Authorisation is sought from the National Veterinary Medicines Agency (Anses-ANMV) or the European Medicines Agency (EMA), ensuring the drug meets stringent safety and efficacy standards.
Consequently, the development process for a veterinary medicinal product is long and complex. Véto-pharma is more committed than ever, investing 8 to 10% of its resources in innovation annually, supported by a dedicated team of around ten experts. Alongside internal projects, Véto-pharma collaborates with external partners like Simon Fraser University in Canada to enhance research into varroa mite treatments, focusing on new active ingredients and advanced galenic technologies. Our primary goal is to empower the beekeeping industry with innovative solutions to ensure beekeepers have the resources they need to succeed.
 by Véto-pharma
by Véto-pharma  by Véto-pharma
by Véto-pharma  by Véto-pharma
by Véto-pharma Join the Véto-pharma community and receive our quarterly newsletter as well as our occasional beekeeping news. You can unsubscribe at any time if our content does not suit you, and your data will never be transferred to a third party!
© 2019-2025, Véto-pharma. All rights reserved