Bees play a fundamental role in the proper functioning of ecosystems through pollination. In recent decades, there has been a decline in pollinator populations in general, and more specifically, honey bees. These losses are due to a combination of factors such as nutritional stress, the incidence of various pathogens and parasites, and exposure to pesticides (Castelli et al., 2020).
Nutritional stress is caused by the increase in monoculture areas, which have reduced the availability of diverse pollen to meet the needs of bees.
At the individual level, feeding on quality pollen is one of the factors that influences longevity. At the colony level, its ingestion by young bees increases the size of the acini in the hypopharyngeal glands (DeGrandi-Hoffman et al., 2010). Its function is key in the secretion of the protein fraction of royal jelly and worker jelly, and consequently in the diet received by larvae, the queen, and the workers that make up the colony (Figure 1).
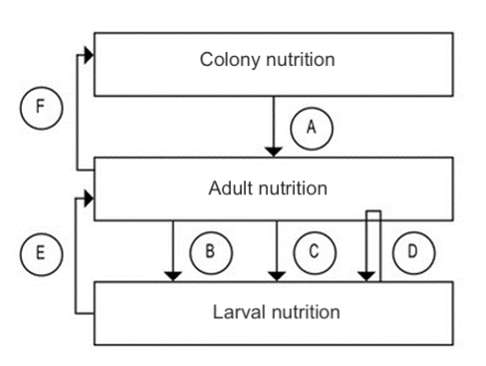
A: dependency of adults on stored food in the colony; B: related to larval quality; C: regulation of the number of larvae; D: cannibalism; E: impact of larval nutrition on the next adult generation; F: impact of adults on colony nutrition. Modified from Brodschneider et al., 2010.
Therefore, the evolution of hypopharyngeal glands correlates with the age of bees and their role within the colony (Corby-Harris et al., 2018). However, the lack of pollen can negatively impact bee populations not only due to the absence of food for nourishing larvae but also in general health (Figure 2), affecting bees’ metabolism, immunity, tolerance to pathogens, and sensitivity to pesticides (Di Pasquale et al., 2013).
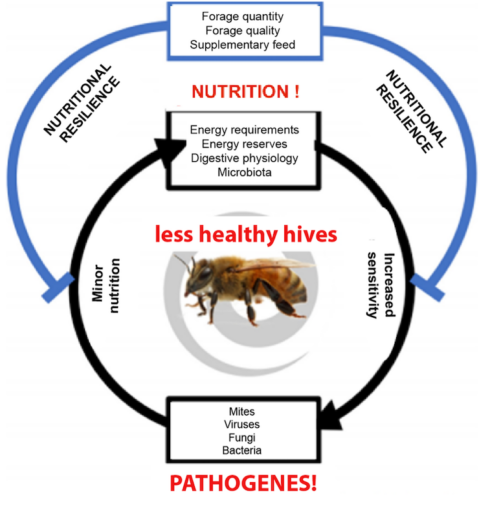
The relationship between immunity and nutrition has been extensively studied in numerous organisms, and bees are no exception. Pollen provides essential amino acids necessary for the synthesis of antimicrobial peptides, activates metabolic pathways, and the expression of genes related to longevity, immune response, and pesticide detoxification. Nectar and honey provide the necessary energy to activate humoral and cellular responses, in addition to containing secondary metabolites with antimicrobial properties (DeGrandi-Hoffman et al., 2015).
Compared to other insects, such as mosquitoes, bees have a simpler immune system, complemented by social immunity. Individually, bees have several barriers that allow them to defend against parasites and pathogens. The exoskeleton or the peritrophic membrane of the intestine would be the first lines of defense. If a parasite or pathogen breaks these barriers, the second line would be the cellular and humoral immune response.
The humoral response is mainly associated with certain antimicrobial peptides, such as abaecin, defensin, or hymenoptaecin. The cellular response is mediated by hemocytes (cells found in the hemolymph) and their response, such as phagocytosis or encapsulation (Negri et al., 2019).
An example of this immune response is the action of antioxidant enzymes, such as phenoloxidase. This enzyme is responsible for the formation of melanin, which the immune system uses for defense. In fact, melanization is considered one of the most important defense responses in adult bees (Negri et al., 2019). Therefore, adequate nutrition is crucial since the synthesis of these enzymes involves a significant energy expenditure and requires essential amino acids that are only acquired through the diet.
This relationship between nutrition and immunity is compromised with the presence of certain parasites, such as varroa. The mite, by feeding on both fat bodies and hemolymph (Han et al., 2024), causes malnutrition, resulting in lower protein levels in parasitized adult bees and pupae (Aronstein et al., 2012). Under these conditions, there is a decrease in protein metabolism, as well as inhibition of immunity-related genes, increasing the virus replication rate (DeGrandi-Hoffman et al., 2015).
Furthermore, nutritional stress also affects the microbiota and, consequently, immunity, as it modulates the expression of genes related to the bee’s immune system. Additionally, it is involved in bee metabolism, growth, development, and defense against pathogens. When a microbiota imbalance (dysbiosis) occurs, bees’ ability to respond to various stress factors is affected (Raymann et al., 2018).
There are numerous studies in the literature evaluating the effect of nutritional stress on bee health. One example is the study conducted by Castelli et al. (2020), which observed that, under laboratory conditions, bees fed with Eucalyptus grandis pollen experienced a microbiota imbalance, affecting both the immune system and pathogen development.
This pollen was selected for its low lipid content and the essential fatty acid omega-3, its deficiency in isoleucine (essential amino acid), and because the protein percentage decreases throughout the flowering period.
Additionally, a reduction in the expression of vitellogenin and immunity-related genes was observed, along with an increase in the multiplication of Nosema ceranae. Vitellogenin is a reserve protein that has multiple functions in bees (embryo nutrition, related to longevity, involved in royal jelly synthesis, etc.). It is used in research to evaluate the nutritional status of bees, as its levels are related to diet quality (Ricigliano et al., 2022).
In another recent study by Corona et al. (2023), the effect of pollen feeding on bee health was evaluated. Under laboratory conditions, two groups were established (including bees of different ages): fed with and without pollen. The group of bees fed with pollen showed higher expression of immune system genes. Various viruses, such as DWV (deformed wing virus), SBV (sacbrood virus), or BQCV (black queen cell virus), were also evaluated. The results showed that both nutritional stress and aging contribute to increased virus levels. Surprisingly, an increase in DWV was observed in the pollen-fed group of bees. These results coincide with those published in other studies (Alaux et al., 2011; Branchiccela et al., 2019), suggesting that increased cellular machinery activity could favor virus multiplication but might also help resist infection.
Sometimes, when there are certain deficiencies or pollen shortages, beekeepers use protein supplements to compensate. Although these are not nutritionally equivalent to pollen, various studies demonstrate a positive effect on colony vigor (Noordyke et al., 2022; Ricigliano et al., 2022). However, there are also studies where no benefits were observed after their application (DeGrandi-Hoffman et al., 2008; Mortensen et al., 2019) or even some where they had a negative impact (DeGrandi-Hoffman et al., 2016). The results depend on many factors, such as the time of application, climatic conditions, the region and location of the apiary, the quality of the food, or the availability of external resources (nectar and pollen).
In recent decades, numerous studies on bee nutrition have been published (Figure 3), and there is increasing knowledge of the nutritional needs of bees and their impact on bee health. Currently, the focus is on using quality dietary supplements to address specific deficiencies and help improve bee health, thereby reducing the use of chemicals for disease management.
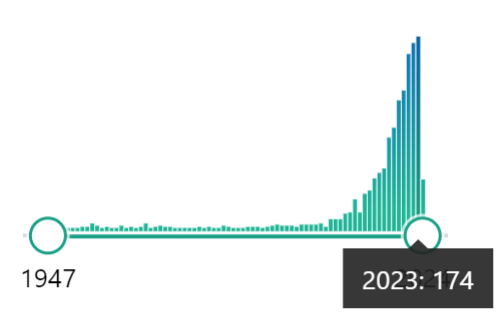
Traditionally, pollen quality has been associated with its protein and amino acid content, and protein supplement formulations have focused on this. Regarding essential amino acids, the work by de Groot in 1953 has served as a basis for establishing minimum dietary requirements to this day. However, new proposals are emerging based on current knowledge. In the study conducted by Ricigliano et al. (2022), it is proposed to establish the ratio of essential amino acids based on leucine, as it is the limiting amino acid for bee growth. Another study by Castaño et al. (2022) suggests adding alanine, asparagine, glycine, and glutamate to protein supplements because, although these are not essential amino acids for adult bees, they are essential for larvae.
In addition to proteins and carbohydrates, recent studies highlight the importance of fats in bee nutrition due to their positive effect on bee health. Among these are essential fatty acids, which must be acquired through the diet, such as linoleic acid (omega-6) and linolenic acid (omega-3) (Figure 4). These fatty acids represent, on average, 43% of the total fatty acids in pollen, and their omega-6
ratio is around 0.8 (Corby-Harris et al., 2021). Both fatty acids are important for bees, as their deficiency leads to reduced development of the hypopharyngeal glands and lower learning capacity (Arien et al., 2015; 2018). Given their importance, it is recommended to consider these nutrients when formulating commercial supplements (Figure 4) (Avni et al., 2014; Corby-Harris et al., 2021).
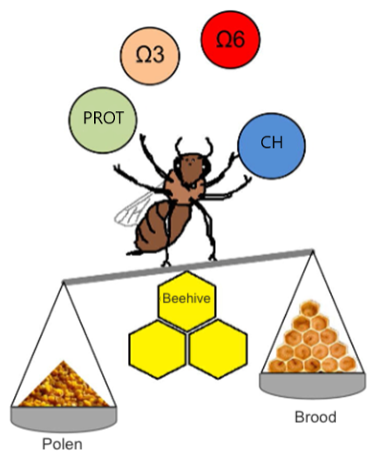
Lately, nutraceutical products such as prebiotics (compounds that promote the growth of microbiota), probiotics (beneficial living organisms), or postbiotics (bioactive compounds produced by probiotics) have been appearing on the international market with the aforementioned objective. There are also products based on plant extracts used in protein patties or syrups that protect against pathogens, such as Nosema spp. (Gajger et al., 2011).
An example of prebiotic use is the employment of spirulina as a pollen substitute. Nutritionally, this microalga is rich in essential amino acids and lipids. According to the study conducted by Ricigliano et al. (2020), its application improved the nutritional status of bees, increasing the expression of vitellogenin, body size, and the abundance of the gut microbiota.
The use of probiotics still requires more research. Recent studies (Damico et al., 2023; Anderson et al., 2024) propose that probiotics formulated with native species of bee microbiota could be effective, while those formulated with bacteria not native to the digestive tract do not seem to be effective.
In summary, although much research remains to be done, good nutrition is fundamental to bee health, as it provides the necessary nutrients for their development, immune function, and disease and parasite resistance.
 by Jose Antonio Babiano
by Jose Antonio Babiano  by Véto-pharma
by Véto-pharma Alaux, C., Dantec, C., Parrinello, H. & Le Conte, Y. 2011. Nutrigenomics in honey bees: Digital gene expression analysis of pollen’s nutritive effects on healthy and varroa-parasitized bees. BMC Genomics. 12, 496 (2011)
Anderson, K. E., Allen, N. O., Copeland, D. C., Kortenkamp, O. L., Erickson, R., Mott, B. M., Oliver, R. 2024. A longitudinal field study of commercial honey bees shows that non‑native probiotics do not rescue antibiotic treatment, and are generally not beneficial. Scientific Reports, 14:1954. https://doi.org/10.1038/s41598-024-52118-z
Arien, Y., Dag, A., Zarchin, S., Masci, T., Shafir, S. (2015). Omega-3 deficiency impairs honey bee learning. PNAS 112 (51) 15761-15766. https://doi.org/10.1073/pnas.151737511.
Arien, Y., Dag, A., Shafir, S. (2018). Omega-6:3 ratio more than absolute lipid level in diet affects associative learning in honey bees. Frontiers in Psychology 19. https://doi.org/10.3389/fpsyg.2018.01001
Aronstein, K. A., Vega, S. E., Westmiller, R., Douglas, S. A. E. 2012. How Varroa parasitism affects the immunological and nutritional staus of the honey bee, Apis mellifera. Insects, 3:601-615.
Avni, D., Hendriksma, H. P., Dag, A., Uni, Z., Shafir, S. 2014. Nutritional aspects of honey bee-collected pollen and constraints on colony development in the eastern Mediterranean. Journal of Insect Physiology, 69:65-73. DOI: 10.1016/j.jinsphys.2014.07.001
Branchiccela, B., Castelli, L., Corona, M., Díaz-Cetti, S., Invernizzi, C., Martínez de la Escalera, G., Mendoza, Y., Santos, E., Zunino, P., Antúnez, K. 2019. Impact of nutritional stress on the honeybee colony health. Scientific Reports. 9:10156. https://doi.org/10.1038/s41598-019-46453-9
Brodschneider, R., Crailsheim, K. 2010. Nutrition and health in honey bees. Apidologie, 41:278-294. DOI: 10.1051/apido/2010012
Castaños, C. E. 2022. The dynamic metabolome of honey bee (Apis mellifera) under starvation and suboptimal nutrition. [Doctoral Thesis, University of Western Australia]. Research Repository-University of Western Australia.
Castelli, L., Branchiccela, B., Garrido, M., Invernizzi, C., Porrini, M., Romero, H., Santos, E., Zunino, P., Antúnez, K. Impact of nutritional stress on honeybee gut microbiota, immunity, and Nosema ceranae infection. Microb. Ecol. 2020, 80, 908–919.
Corby-Harris, V., Bennet, M. M., Deeter, M. E., Snyder, L., Meador, C., Welchert, A. C., Hoffman, A., Obernesser, B. T., Carroll, M. J. 2021. Fatty acid homeostasis in honey bees (Apis mellifera) fed commercial diet supplements. Apidologie, 52:1195-1209.
Corby-Harris, V., Snyder, L. A. 2018. Measuring hypopharyngeal gland acinus size in honey bee (Apis mellifera) workers. J. Vis. Exp. 139, e58261, doi:10.3791/58261
Corona, M., Branchiccela, B., Alburaki, M., Palmer-Young, E. C., Madella, S., Chen, Y., Evans J. D. 2023. Decoupling the effects of nutrition, age, and behavioral caste on honey bee physiology, immunity, and colony health. Front. Physiol. 14:1149840. doi: 10.3389/fphys.2023.1149840
Damico, M. E., Beasley, B., Greenstein, D., Raymann, K. 2023. Testing the effectiveness of a commercially sold probiotic on restoring the gut microbiota of honey bees: a field study. Probiotics and Antimicrobial Proteins, DOI: 10.1007/s12602-023-10203-1
DeGrandi-Hofman, G., Chen, Y. 2015. Nutrition, immunity and viral infections in honey bees. Current Opinion in Insect Science, 10:170-176. http://dx.doi.org/10.1016/j.cois.2015.05.007
DeGrandi-Hoffman, G., Chen, Y., Huang, E., Huang, M. H. 2010. The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.). Journal of Insect Physiology. 56(9):1184-1191
DeGrandi-Hoffman, G., Chen, Y., Rivera, R., Carroll, M., Chambers, M., Hidalgo, G., de Jong, E. W. 2016. Honey bee colonies provided with natural forage have lower pathogen loads and higher overwinter survival than those fed protein supplements. Apidologie, 47, 186–196. https://doi.org/10.1007/s13592-015-0386-6
DeGrandi-Hoffman, G., Wardell, G., Ahumada-Segura, F., Rinderer, T., Danka, R., Pettis, J. 2008. Comparisons of pollen substitute diets for honey bees: Consumption rates by colonies and effects on brood and adult populations. Journal of Apicultural Research, 47, 265–270. https://doi.org/10.3896/IBRA.1.47.4.06
Di Pasquale, G., Salignon, M., Le Conte, Y., Belzunces, L. P., Decourtye, A., Kretzschmar, A., Suchail, S., Brunet, J. L., Alaux, C. 2013. Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS ONE 8(8): e72016. doi:10.1371/journal.pone.0072016
Dolezal, A. G., Toth, A. L. 2018. Feedbacks between nutrition and disease in honey bee health. Current Opinion in Insect Science, 26:114-119. https://doi.org/10.1016/j.cois.2018.02.006
Gajger, I. T., Kozaric, Z., Berta, D., Nejedli, S., Petrinec, Z. 2011. Effect of the herbal preparation Nozevit on the mid-gut structure of honeybees (Apis mellifera) infected with Nosema sp. spores. Veterinarni Medicina, 56(7):344-351
Han, B., Wu, J., Wei, Q., Liu, F., Cui, L., Rueppell, O., Xu, S. 2024. Life-history stage determines the diet of ectoparasitic mites on their honey bee hosts. Nature Communications, 15:725. https://doi.org/10.1038/s41467-024-44915-x
Mortensen, A. N., Jack, C. J., Bustamante, T. A., Schmehl, D. R., & Ellis, J. D. 2019. Effects of supplemental pollen feeding on honey bee (Hymenoptera: Apidae) colony strength and Nosema spp. infection. Journal of Economic Entomology, https://doi.org/10.1093/jee/toy341
Negri, P., Villalobos, E., Szawarski, N., Damiani, N., Gende, L., Garrido, M., Maggi, M., Quintana, S., Lamattina, L., Eguaras, M. 2019. Towards precisión nutrition: a novel concept linking phytochemiclas, immune response and honey bee health. Insects, 10(11):401. https://doi.org/10.3390/insects10110401
Noordyke, E. R., van Santen, E., Ellis, J. D. 2021. Evaluating the strength of western honey bee (Apis mellifera L.) colonies fed pollen substitutes over winter. Journal of Applied Entomology, 146:291-300. DOI: 10.1111/jen.12957
Raymann, K., Moran, N. A. 2018. The role of the gut microbiome in health and disease of adult honey bee workers. Current Opinion in Insect Science, 26:97-104.
Ricigliano, V., Simone-Finstrom, S. 2020. Nutritional and prebiotic efficacy of the microalga Arthrospira platensis (spirulina) in honey bees. Apidologie, 51:898-910.
Ricigliano, V. A., Williams, S. T., Oliver, R. 2022. Effects of different artificial diets on commercial honey bee colony performance, health biomarkers, and gut microbiota. BMC Veterinary Research, 18:52. https://doi.org/10.1186/s12917-022-03151-5
Join the Véto-pharma community and receive our quarterly newsletter as well as our occasional beekeeping news. You can unsubscribe at any time if our content does not suit you, and your data will never be transferred to a third party!
© 2019-2025, Véto-pharma. All rights reserved