This case study, “Honey Bee Brood Disease,” was submitted by Dr. Caroline Lantuejoul, a veterinarian specializing in beekeeping and apicultural diseases in France.
It presents a conventional beekeeping operation facing health challenges in a nuc apiary. The operation, comprising approximately 400 hives spread across 36 apiaries, produces over 10 tons of honey and 150 nucs annually.
Clinical examinations revealed brood anomalies in several colonies, including mosaic and bald brood, as well as larval deformities. Laboratory analyses confirmed the presence of European foulbrood (EFB) combined with high viral loads (DWV-B, SBV, BQCV), despite controlled varroa management and low varroa infestations during the clinical evaluation.
These health issues are likely multifactorial, linked to unfavorable weather conditions that caused a prolonged nectar dearth, potentially worsened by pollen shortages. Management included transferring affected colonies, heightened monitoring, and strict hygiene measures.
This study emphasizes the importance of a holistic approach to bee health management, considering environmental, nutritional, and pathogenic factors, even in well-managed operations.
The beekeeper in question is a professional operating a conventional farming system. Two full-time employees work on the operation, focusing primarily on honey production with minor ancillary activities.
In 2023, the operation included around 400 hives spread across 36 apiaries. The production infrastructure supports the annual production of six to seven types of honey, often exceeding the annual target of 10 tons, with outputs of 13.5 tons in 2023, 15.35 tons in 2022, and 13.24 tons in 2021. Transhumance practices involve moving 80 to 160 hives to exploit blooming crops like rapeseed, acacia, forest flora, and sunflower. In some years, these transhumance hives alone have produced up to 5 tons of honey.
Additionally, approximately 150 nucs are produced yearly to replenish stock. The beekeeper uses Buckfast queens and strains from recognized breeders, with an annual renewal rate of 50-85% for young queens.
The visit was prompted by health concerns involving brood anomalies in a nuc apiary. Two weeks prior, the beekeeper reported a catastrophic health state in the apiary, during a nectar dearth caused by adverse weather conditions.
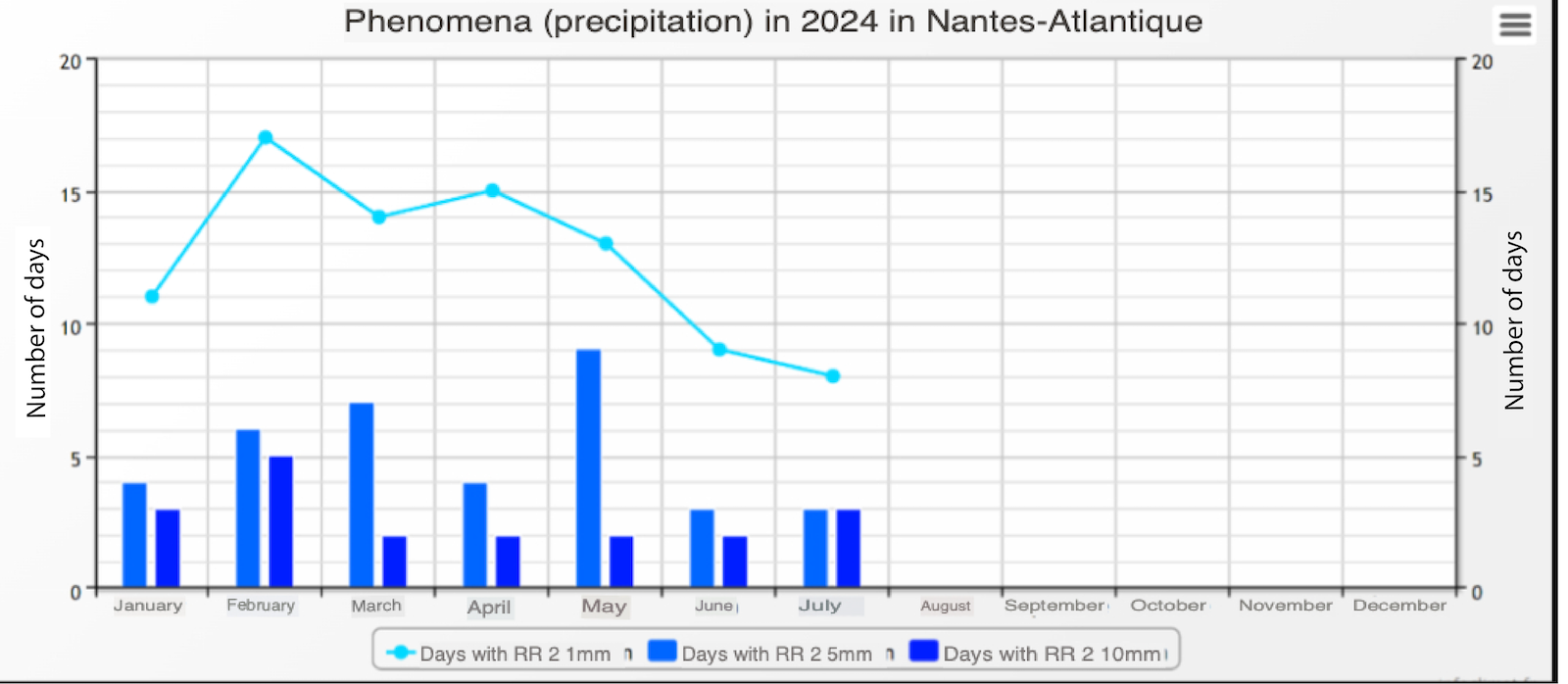
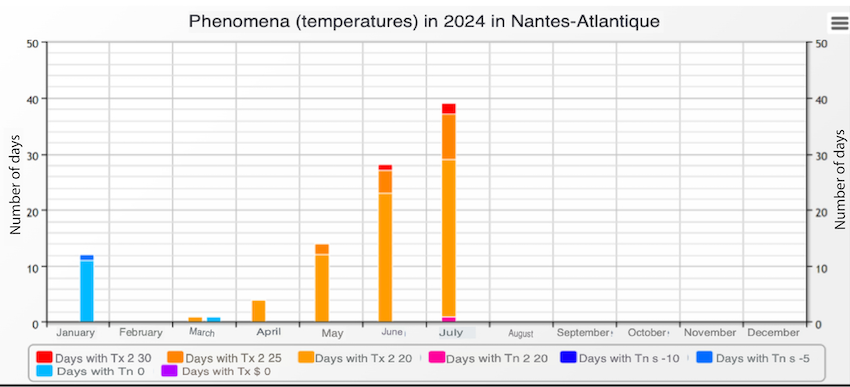
Spring had been particularly rainy, with 13-17 rainy days per month since the start of the season. Colony activity resumed early due to the absence of winter frosts, but daily temperatures rarely exceeded 20°C (68°F) (see local weather data below).
These weather conditions severely restricted foraging, especially on rainy days, and limited food resources, as low temperatures reduced nectar availability. This atypical weather pattern affected the entire western France region.
For varroa mite management, the beekeeper typically employs a dual therapy approach with a conventional summer treatment (a veterinary drug based on amitraz) and a winter broodless treatment (a veterinary drug based on oxalic acid) applied by dribbling. Until 2024, no monitoring of varroa mite infestation levels had been implemented. Feeding is carried out as needed using commercially available syrup, and equipment is cleaned (scraping or washing) and disinfected (using a flame or hot soda bath). Wax is produced in-house from capping wax, with strict rotation practices.
The apiary affected by the health issue comprises 23 wooden Dadant nucs with 6 frames, located in the countryside near a marsh. The nucs were established in the spring with the introduction of J10 queen cells from the operation’s own breeding stock, grafted onto a purchased strain (three different strains were used). The nucs were treated with oxalic acid (a licensed veterinary drug) during the broodless period at the time of egg-laying checks. This year, three rounds of syrup feeding (about 1 kg per round) were necessary due to challenging nutritional conditions. The colonies have been able to take advantage of the blackberry honey flow for about 15 days.
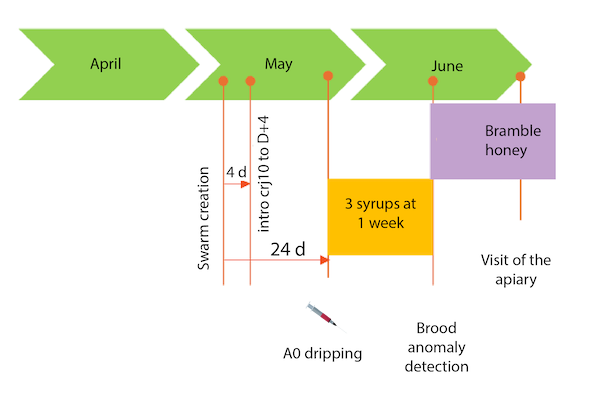
During the visit, the wooden Dadant nucs (6 frames) were well-maintained, although the apiary grounds had yet to be cleared—a task planned after the queen fertilization period. The colonies were distributed across six pallets, each holding three or four nucs.
Colony Inspections
Before opening the hives, no anomalies were observed. Foraging activity was intense across the apiary, aided by favorable weather on the visit day. Upon inspection, most colonies were populous, with bees in feeders. Honey supers were set to be added soon. Pollen and honey reserves were satisfactory, and nectar collection indicated an ongoing blackberry flow. Colonies occupied 4-6 brood frames.
Varroa Infestation Monitoring
Varroa mite counts on phoretic bees were conducted using CO₂, sampling eight colonies randomly across the apiary. Results were satisfactory:
Laboratory Sampling
Samples were taken for lab analyses:
Samples from Colonies 2b and 5a were initially analyzed at LDA39 (Laboratoire Départemental d’Analyses du Jura) dans un premier temps.
Below are the thresholds validated by the ANSES laboratory concerning viral analyses. These thresholds were established based on analyses conducted on general worker bees. There is no available data regarding analyses performed on brood.
For colony 2b :
Laboratory analyses of the brood confirm the clinical presence of European foulbrood. Viral loads detected in the brood for BQCV (Black Queen Cell Virus) and SBV (Sacbrood Bee Virus) are reported as insignificant. The viral load for DWV-B (Deformed Wing Virus type B) is noted to be near the clinically accepted threshold for bees.
Analyses conducted on asymptomatic interior bees from Colony 2b yielded the following results:
For colony 5a :
Analyses conducted on the brood of Colony 5a:
Analyses conducted on asymptomatic interior bees from Colony 5a :
At the conclusion of the visit, one colony appeared to be affected by atypical European foulbrood, and clinical signs of viral diseases were also present in the apiary. Laboratory analyses confirmed both atypical European foulbrood and viral infections despite good control of varroa mite pressure.
The colonies were populous and, since the blackberry honey flow and improved weather, appeared to be cleaning their brood.
For the colony clinically affected by European foulbrood, which was very populous, it was decided to perform a sanitary transfer onto new wax. Following the analyses, other colonies with brood that appeared unhealthy were also transferred to prevent any risk of resurgence or contamination of other colonies.
It was decided not to replace the queens initially due to the highly erratic weather conditions this season.
Heightened monitoring of the apiary was maintained throughout the season, with all colonies confined to the same site.
A systematic “work from clean to contaminated” approach was implemented during inspections, opening the healthiest colonies first and progressing to the most affected ones. Tools and gloves were rigorously cleaned and disinfected to minimize contamination risks.
The issues observed in this apiary are part of a complex environmental and health context, highlighting the delicate interplay between nutritional, pathogenic, and climatic factors affecting honey bee colony health.
The exceptionally rainy season significantly hindered foraging activity, leading to a prolonged nectar dearth. Although supplemental feeding was implemented, it is likely that nutritional deficiencies, particularly in pollen, contributed to the health problems observed. This hypothesis aligns with the findings of Di Pasquale et al. (2013), who demonstrated the critical impact of pollen diversity and quality on honey bees’ resistance to pathogens.1
The atypical condition of the apiary may be explained by a pathogenic synergy involving viruses and Melissococcus plutonius (European foulbrood). This harmful association, exacerbated by unfavorable environmental conditions, illustrates the complexity of pathogen interactions in honey bees, as noted by Nazzi and Pennacchio (2018) in their review on multiple stress factors affecting bee health.2
The high levels of viral infections, despite satisfactory Vvarroa mite management, raise questions about the mechanisms of virus transmission and persistence in colonies. Recent studies, such as those by Remnant et al. (2019), have highlighted the ability of certain viruses to persist in bee populations independently of varroa infestation levels.3
The prolonged confinement of populous nucs may have exacerbated viral propagation within the colonies, a phenomenon described by Beaurepaire et al. (2020) in their study on viral transmission dynamics in overcrowded hives.4
The lack of data on the parasitic and viral loads of parent hives represents a limitation of this case study. Additional analyses could have provided valuable insights into the origin and progression of the health issues observed, as emphasized by Thaduri et al. (2018) in their research on vertical virus transmission in bees.5
—
In conclusion, this clinical case underscores the necessity of a holistic approach to apiary health management, taking into account the complex interactions between nutrition, pathogens, and the environment. It also highlights the importance of continuous monitoring and in-depth analyses to achieve a comprehensive understanding of the health dynamics within honey bee colonies.
References :
Table of Contents In this article, we present two clinical cases of Chronic Bee Paralysis Virus (CBPV) from Spain. We extend our gratitude to Ana Mompó and Inma Segura (ADS
 by Gérald Therville
by Gérald Therville  by Juan Molina
by Juan Molina Join the Véto-pharma community and receive our quarterly newsletter as well as our occasional beekeeping news. You can unsubscribe at any time if our content does not suit you, and your data will never be transferred to a third party!
© 2019-2025, Véto-pharma. All rights reserved