Each year, several thousand of you use treatments to combat Varroa destructor. These treatments are based on different active substances (amitraz, oxalic acid, thymol, formic acid, tau-fluvalinate, etc.) and offer several methods of action and administration routes (rapid action, long action, contact, trickling, sublimation, etc.). Yet, it’s often forgotten that a mite treatment is, first and foremost, a veterinary medicine. And that veterinary medicines must answer particular constraints and processes inherent to their status. This subject, although interesting, is often little known.
A veterinary medicine is a formulation based on an active substance that has received a marketing authorisation from the competent authorities (national veterinary agencies or the European Medicine Agency [EMA]). Several years are necessary between the start of Research & Development and the Marketing Authorisation. During this time the laboratory will complete various steps (search for an active substance, in-vitro tests, clinical data [test in “field” conditions]) to arrive at the marketing authorisation application phase.
To obtain this final authorisation, the future Marketing Autorization Holder must prove the quality, efficacy, and safety (non-harmful effect) of the formulation. To that end, it will have to submit a Market Authorisation application, a lengthy and costly procedure containing many studies:
Once these steps have been completed, the national or European agency validates a “SPC” (Summary of Product Characteristics) which summarises all the drug’s characteristics (posology, instructions for use, precautions, secondary effects, etc.) and which also defines its framework of use (in particular posology, precautions, and duration of application).
The commitment of Véto-pharma for honey bee health started 25 years ago, when we registered our first varroa mite treatment back in 1995.
Manufacturing a varroa mite treatment is not an easy process and cannot be made in any production site. The Véto-pharma manufacturing plant is GMP certified (Good Manufacturing Practices), which guarantees compliance with European directives and their quality standards. Indeed, it is only possible to manufacture a drug in structures that hold a pharmaceutical manufacturing authorisation. GMP are the principles and guidelines to be followed for manufacturing drugs for human and veterinary use. During manufacturing, the same standards are applied to the drug, whether it is for human or veterinary use.

Each batch of product is subject to many quality tests at each production step:
All these checks are noted in the “batch record” as production progresses. At the end of production, the Qualified Person reads the entire record and validates whether the batch will be sold or not. If the batch is declared non-conform, it will be removed and destroyed.
After “releasing” the batches, these vet medicines will be shipped only in the countries where they received an authorization by the local veterinary medicine agencies.
Oxybee is the most recent addition to our portfolio. It became apparent that beekeepers needed an organic mite treatment that they could especially use in winter (use in brood free colonies only) as a complementary treatment for the hives presenting a high infestation. We were committed to meeting this need. To make this product available to European beekeepers, a centralised registration was carried out through the European Medicine Agency.

The key advantage of Oxybee is its innovative formulation. The combination of sucrose and glycerol in the Oxybee formulation enables a better distribution of oxalic acid solution in the colony.1-2 The formation of small droplets of solution that last longer in the colony (increased hygroscopy) is assumed to be the cause of this effect, enabling a better distribution of oxalic acid solution in the hive.1-2 As a result, field data have shown a higher efficacy of Oxybee compared to a standard formulation of oxalic acid and sucrose.3

How will varroa be treated in the future? There are few products currently available that combine a satisfactory level of effectiveness with limited use constraints for beekeepers. But we are aware of the urgent need to develop new active ingredients that will make it possible for varroa to be sustainably controlled, and for treatments to be rotated risk-free.
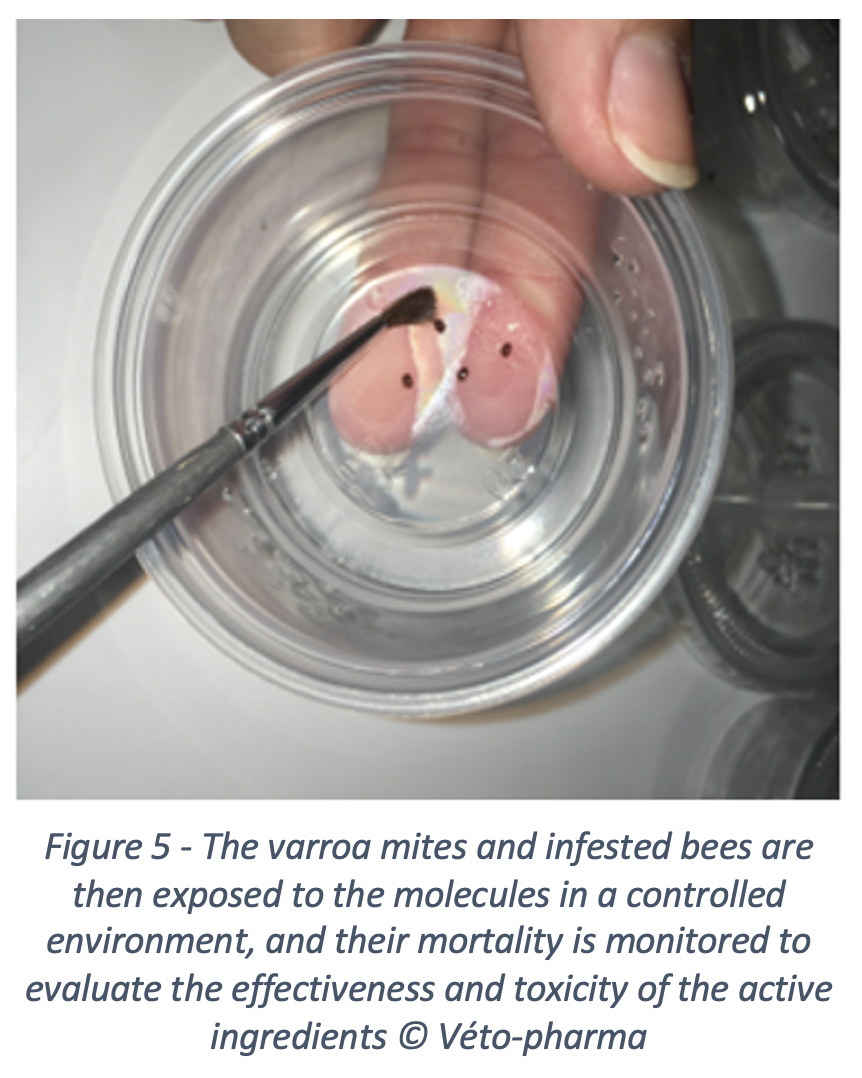
To achieve this, Véto-pharma has invested in innovation for many years. We created the Véto-pharma’s experimental apiary five years ago, along with a bee laboratory. This laboratory houses one of the company’s major innovation projects: screening new active ingredients to fight varroa. For this purpose, the innovation team developed a unique screening method, which makes it possible to evaluate active ingredients and identify the ones that would be effective against varroa, and safe for the colonies. Not an easy feat! Molecules effective against varroa, whether chemical or organic in origin, are often highly toxic for bees. This is particularly true for many essential oils, which only achieve optimal effectiveness at levels that are very harmful to bees.
Over the past years, we evaluated 38 new molecules, and some of them showed promising results. These molecules are currently being re-evaluated and will probably lead, if they pass all the tests (efficacy, safety, residues), to the new treatments of tomorrow.
To learn more about Véto-pharma and our products, please visit www.veto-pharma.eu
References:
1 – CVMP assessment report for Oxybee (EMEA/V/C/004296/0000) – 2017
2 – Milani (2001) – Activity of oxalic aid and citric acids on the mite Varroa destructor in laboratory assays – Apidologie 32 (2001) 127–138 © INRA/DIB-AGIB/EDP Sciences, 2001
3 – Poster G. Braun et al., DVG-Fachgruppentagung “Parasitologie und parasitäre Krankheiten”, Hannover, Germany, Juni 12-14, 2017.
Legal notices:
OXYBEE powder and solution for 39,4 mg/ml bee-hive dispersion for honey bees. Composition: 1 ml of mixed bee-hive dispersion contains 39,4 mg of oxalic acid dehydrate. Indication(s) for use: For the treatment of varroosis (Varroa destructor) of honey bees (Apis mellifera) in brood free colonies. Withdrawal period(s): Honey: zero days. Do not use during honey flow. Special precautions: This veterinary medicinal product is highly acidic and could have irritating and corrosive effects on the skin, eyes and mucous membranes. Personal protective equipment consisting of protective clothing, acid-proof gloves and safety glasses should be worn. Marketing authorisation holder: Dany Bienenwohl GmbH, Geyerspergerstr. 27, 80689 Munich, Germany. Distributed by: Véto-pharma, 12-14 avenue de la Croix Martre 91120 Palaiseau, France. V0119
Oxybee is a veterinary medicine. Please ask advice to your veterinarian, pharmacist or sanitary organization. In case of persistence of clinical signs, consult with your veterinarian. Read carefully the instructions on the product label before use.
VTP-50-EU-N01-01/21
Join the Véto-pharma community and receive our quarterly newsletter as well as our occasional beekeeping news. You can unsubscribe at any time if our content does not suit you, and your data will never be transferred to a third party!
© 2019-2025, Véto-pharma. All rights reserved